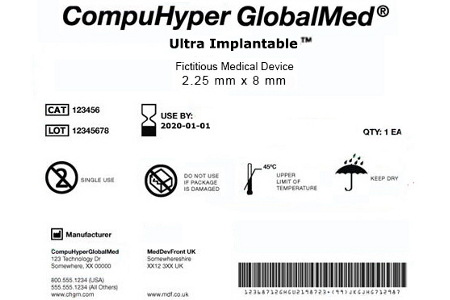
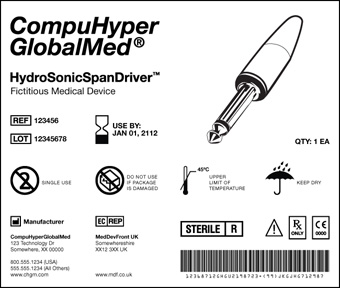
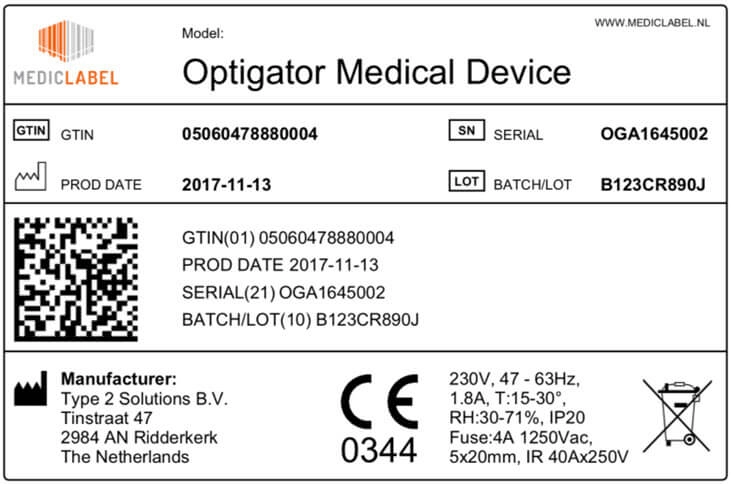
PLOS ONE: Evaluating Varied Label Designs for Use with Medical Devices: Optimized Labels Outperform Existing Labels in the Correct Selection of Devices and Time to Select

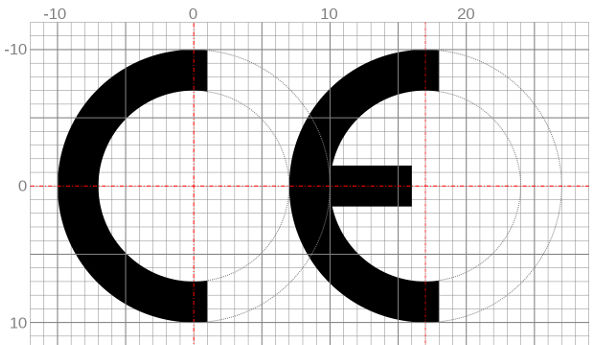
What Does the CE Mark Mean, and What is its Purpose? - Medical Device Academy Medical Device Academy
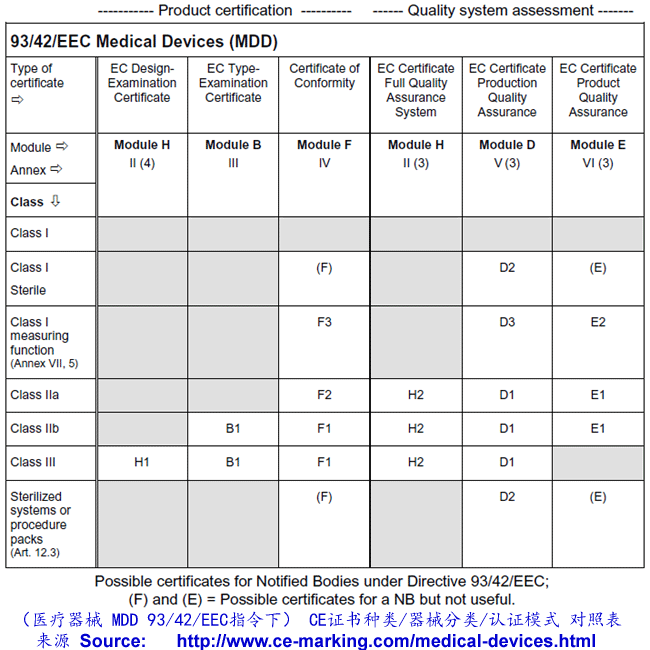
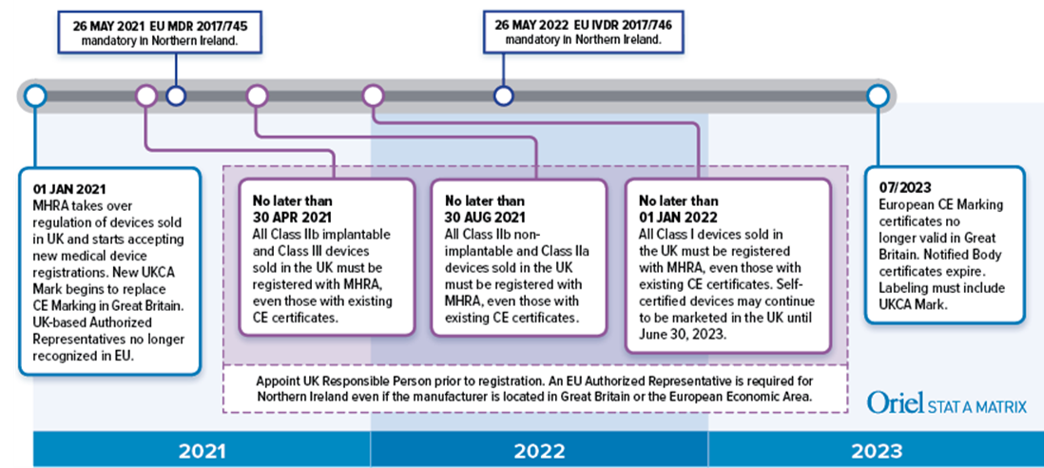
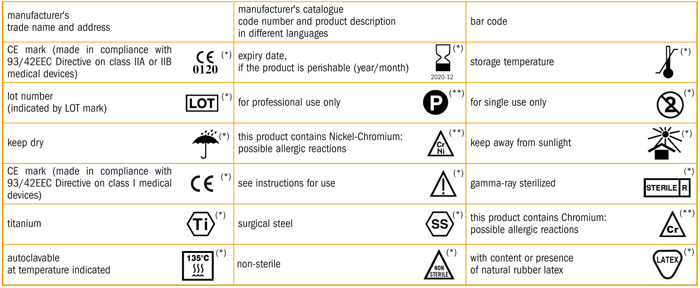
Guide on medical devices (MD/IVD) CE marking (mark) & European (EEA/EU/EC) UK Authorized Representative service in both UK (England) & EU (Ireland).